Quick Summary
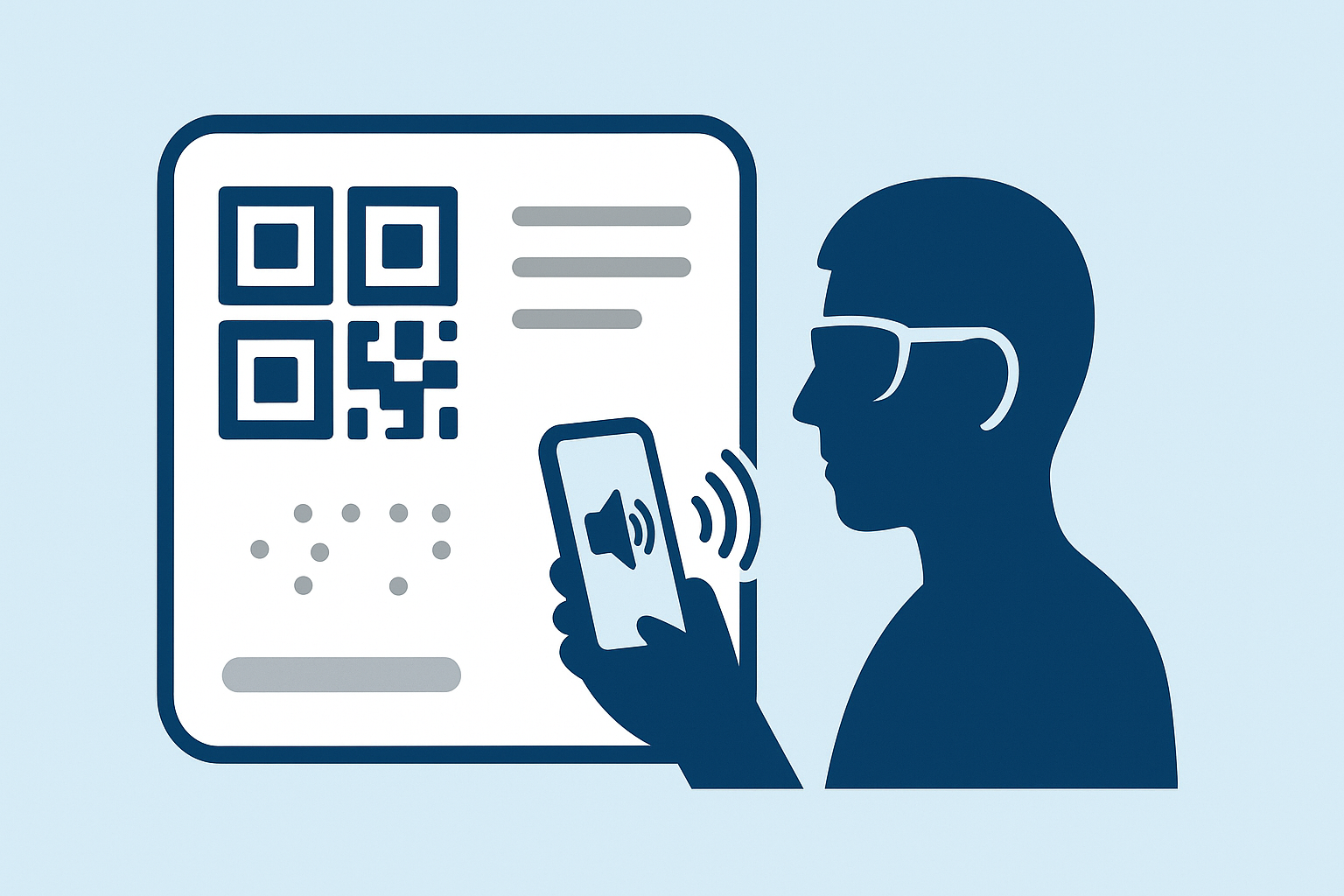
India's drug regulator is weighing a landmark accessibility rule that would add braille inscriptions and voice-assisted QR codes to medicine packaging, giving visually impaired patients independent access to critical health information.
Table of Contents
1. What's Being Proposed?
- Add braille labels on medicine cartons or strips so that visually impaired patients can independently read medicine names and expiry dates.
- Introduce voice-assisted QR codes that, when scanned with a smartphone, play audio covering the medicine's name, dosage, and other safety information.
- Allow voluntary adoption in the initial phase, particularly for specific pack sizes, before moving toward wider implementation.
2. Background & Why It Matters?
Representatives from the visually impaired community have long highlighted the difficulty of reading small print on medicine packages. In response, the Central Drugs Standard Control Organisation (CDSCO) and the Drugs Controller General of India (DCGI) tabled the issue in 2020, asking the Drugs Consultative Committee (DCC) to examine practical solutions. The initiative is now moving toward formal consultation, marking a significant push for inclusive healthcare.
3. Key Recommendations from the DCC Sub-Committee
- Issue detailed guidelines for braille labelling on mono-carton pack sizes.
- Prioritise medicines commonly used by visually impaired patients, such as ophthalmic preparations.
- Exclude categories administered only under supervision (injectables, vaccines) from the initial rollout.
4. Which Products Are in Scope & Which May Be Exempt?
| Category | Likely In Scope | Likely Exempt / Deferred |
|---|---|---|
| Medicines in mono carton pack sizes | Voluntary braille and audio QR codes in the first phase | — |
| High-use substances among visually impaired users (for example, eye-drops) | Priority for implementation | — |
| Medicines dispensed under medical supervision (injectables, vaccines) | — | Expected to remain exempt initially |
5. Process & Public Feedback
The CDSCO and DCGI have opened the proposal for public comment. Stakeholders including patient advocacy groups, pharmaceutical manufacturers, healthcare professionals, and technology partners are encouraged to submit feedback covering feasibility, cost, training needs, and realistic implementation timelines.
6. Potential Impacts & Challenges
Positive Outcomes
Empowers visually impaired patients, reduces medicine mix-ups, and strengthens trust in self-managed care.
Key Challenges
Manufacturing costs, standardising braille formats, ensuring QR audio in local languages, and monitoring compliance across a vast pharma ecosystem.
7. Role of QR Codes in Enhancing Accessibility
QR codes give regulators and manufacturers a flexible way to share dynamic information. Voice-assisted QR solutions can play medicine details aloud, link to instructional videos, provide multilingual support, and push notifications if safety alerts change. For Enqode QR, the proposal underscores how secure, adaptive QR technology can make accessibility regulations practical on the ground.
8. Conclusion
India is moving toward a more inclusive medicine supply chain. By embracing braille labelling and audio-enabled QR codes, the healthcare system can empower visually impaired citizens with autonomy and confidence. The consultation window is a pivotal moment for pharmaceutical firms, QR technology providers, and patient advocates to collaborate on a rollout that is both compassionate and feasible.